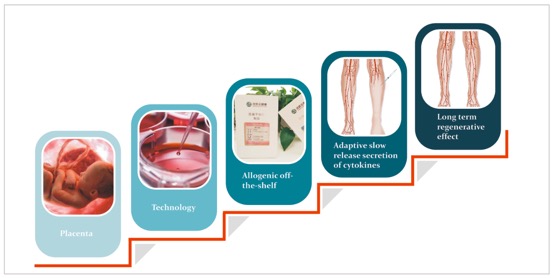
hP-MSC injection


hP-MSC injection is mainly comprised of mesenchymal stromal/stem cells from fetus associated structure at birth, the planceta. Planceta mesenchymal stromal/stem cells (P-MSC), specifically CD106 positive P-MSC which is our proprietary isolation technology, display unique immunomodulatory properties and have powerful potential to induce angiogenesis, especially in the treatment of critical limb ischemia and aplastic anemia. Furthermore, P-MSC transplantation is effective in reducing insulin doses and controlling glycemic level in type 2 diabetes patients. 
-
Indications:Severe lower limb ischemia, autoimmune disease, Type II diabetes.
-
Clinical trial: in June 2018, the application of clinical trial of gradeⅠnew medicine was submitted to the China Food and Drug Administration (CFDA).
-
Clinical study: After published the first clinical research paper in 2011, a series of clinical studies of hP-MSC injection for the treatment of various diseases have been conducted.
-
Pre-clinical study: The pre-clinical safety study was completed in 2011, the inspection report of the National Institutes for Food and Drug Control (NIEDC) was obtained
-
R&D progress: In 2011, the method of discriminating and separating highly active MSC subgroups was invented, and the invention patent was authorized in 2015