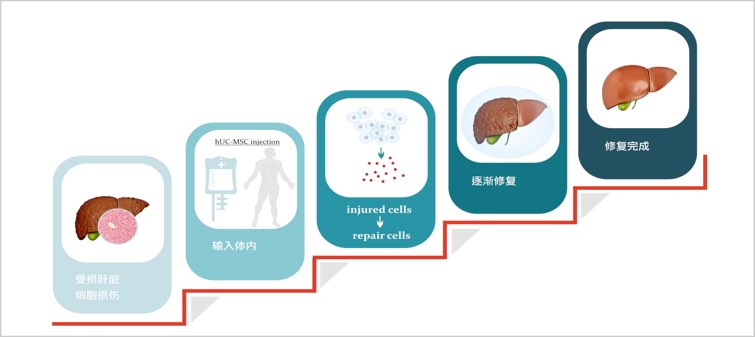
hUC-MSC injection is mainly constructed with mesenchymal stromal/stem cells from fetus associated structure at birth, the umbilical cord. Umbilical cord mesenchymal stromal/stem cells share a number of therapeutic features which enable their use for clinical applications, especially in the treatment of GvHD, liver cirrhosis, myocardial ischemia, and so on.

-
Indications: blood diseases (GvHD, AA), liver injury and liver cirrhosis
-
Clinical trial: in March 2018, the application of clinical trial of gradeⅠnew medicine was submitted to the China Food and Drug Administration (CFDA).
-
Clinical study: After publishing the first clinical research paper in 2009, a series of clinical studies of hUC-MSC injection for the treatment of various diseases have been conducted.
-
Pre-clinical study: The pre-clinical safety and efficacy study was completed in 2006, the inspection report of the National Institutes for Food and Drug Control (NIEDC) was obtained, and the clinical trial of new medicine was formally applied.
-
Stem cell bank: the first umbilical cord mesenchymal stem cell bank and the first technical standard in the world were set up by us in 2006.
-
R & D time: patent application in 2005 and the first research paper published in 2006
Comparison with bone marrow MSC products
-
1. Young donors & rich in MSC number;
-
2. Unlimited source & easy to collect;
-
3. Ethically accepted;
-
4. Highly potent in immunoregulation & pro-angiogenesis;
-
5. Low immunogenicity;
-
6. Easy for large-scale manufacturing;
Liver cirrhosis map