Human placental mesenchymal stem cell gel
1. Caesarean scar case
"hP-MSC hydrogel”is an independently developed product by H&B which has been used in the repair of cesarean scar. the project hosted by Liu Zhengping, director of Foshan maternal and child obstetrics , has successfully registered at the NIH Clinical Trial Center. the registration number is NCT02772289. Up to now, there have been 48 cases of pregnant women included into this registered clinical research. the average cesarean section time is about 38 minutes, the incision length is about 14.6cm, . 7 cases were followed up for 6 months, 10 cases were followed up for 3 months, 21 cases were followed up for 1 months, 10 patients under the age of 1 months. No case was found abnormal reaction. serological markers and tumor markers were all normal, and achieved ideal initial results in safety and effectiveness.
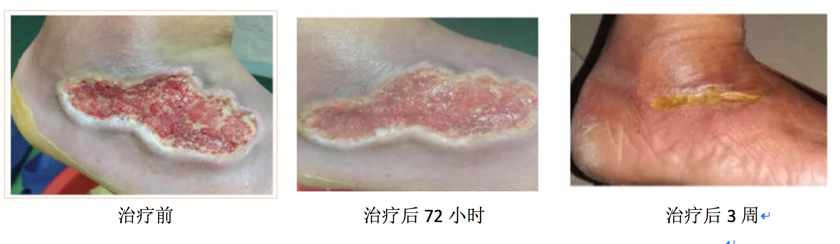
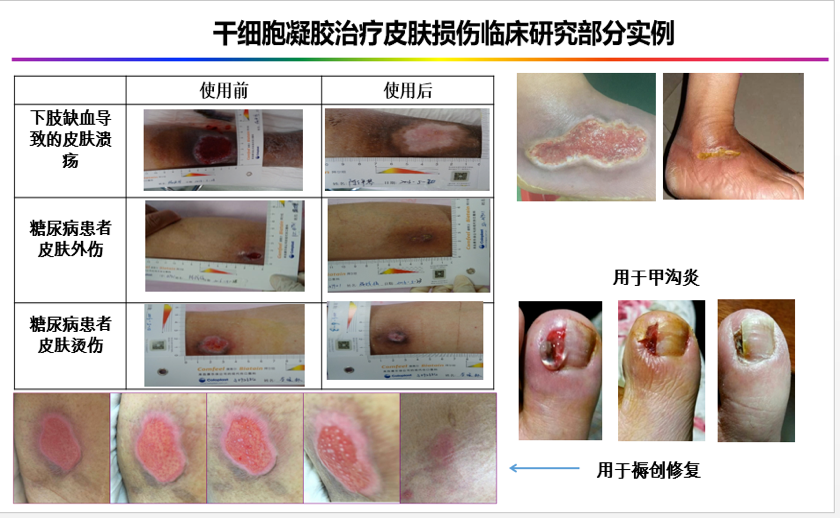
2. diabetic foot ulcers
Effect of placenta derived mesenchymal stem cells gel on diabetic foot ulcers